|
|
 |
 |
|
Refrigeration Cycle
|
Outline
|
|
|
| 1. |
Basic theory of cooling
|
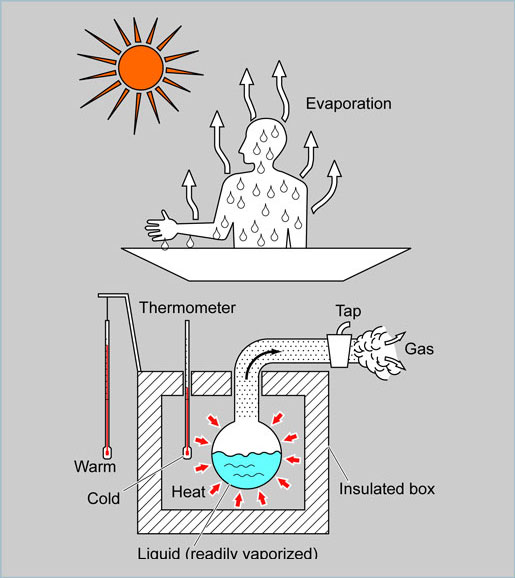
Immediately after leaving the water when you have been swimming, it is common to feel a little cold, even if the temperature of the day is quite high (hot). This is because the water on the surface of your skin is evaporating, partly by “taking away” some of your body heat.
For the same reason, we also feel a cool spot if we apply an alcohol swab to our arm because the alcohol takes away some of the heat from the arm when it evaporates. We can make objects cooler by using these natural phenomena, e.g. causing a liquid to take the heat from a substance when it evaporates.
In this example, a container with a tap is placed inside a well-insulated box. A liquid that will vaporize readily at atmospheric temperature is placed into the container.
As the tap is opened, the liquid in the container will “take away” the heat necessary for vaporization from the air inside the box while turning into a gas and escaping past the tap to the outer side.
While the vaporization of the liquid is occurring, the temperature of the air inside the box will decrease from the point before the tap was opened. |
|
(14)
|
|